12+ Chapter 7 Ionic And Metallic Bonding Answer Key
Also valence shell electron pair repulsion theory or VSEPR theory had limited applications and also failed in predicting the geometry corresponding to complex molecules. Web Gradescope allows me to give a short quiz every day in my section of 60 students and grade them all on my 30 minute train ride home.

Pdf Metal Ion Binding To The Amyloid B Monomer Studied By Native Top Down Fticr Mass Spectrometry
These NCERT Solutions for Class 12 Chemistry.

. UPSC Prelims 2022 Answer Key. Web That being said here we are providing a set of NCERT Solutions for Class 11 Chemistry Chapter 9 to help students test their knowledge about the chapter which is hydrogen and they can also learn how to work on the questions as well. The atomic radii or the ionic radii of elements increases while progressing down a group.
Web A footnote in Microsofts submission to the UKs Competition and Markets Authority CMA has let slip the reason behind Call of Dutys absence from the Xbox Game Pass library. C A covalent bond present between N and C Atom and ionic bond present between Na ion and NC ion. NCERT Solutions for Class 12 Chemistry are drafted by the faculty at BYJUS to help students learn all the complex concepts efficientlyEach and every question from the NCERT Textbook is answered in a systematic format to help students learn in a shorter duration.
Web Microsoft pleaded for its deal on the day of the Phase 2 decision last month but now the gloves are well and truly off. The results of the above two experiments show that an induced emf is produced whenever there is a change in magnetic flux caused by a. Web NCERT Solutions for Class 10 Maths Chapter 7.
NCERT Solutions for Class 10 Maths Chapter 8. NCERT Solutions for Class 12 Chemistry Chapter 8 The d and f Block Elements are powerful study materials that have answers to textbook exercises and important questions from previous years and sample papers. Covalent bonds are.
Web The latest Lifestyle Daily Life news tips opinion and advice from The Sydney Morning Herald covering life and relationships beauty fashion health wellbeing. NCERT Solutions for Class 10 Maths Chapter 12. Web NCERT Solutions for Class 12 Chemistry Chapter 8 Free PDF Download.
UPSC Prelims 2022 Answer Key. A current is induced in an electric circuit whenever the magnetic flux linked with the circuit keeps on changing either as a result of changing current in a nearby circuit or due to relative motion between them. Web Organometallic Chemistry of the s- and p-block elements.
Classification of Elements and Periodicity in Properties Class 11 Important Questions. Long Answer type Questions. Once dissolved or melted ionic compounds are excellent conductors of electricity and heat because the ions can move about freely.
Web where A is the Hamaker coefficient which is a constant 10 19 10 20 J that depends on the material properties it can be positive or negative in sign depending on the intervening medium and z is the center-to-center distance. The electrostatic force of attraction which holds the two oppositely charged ions together is called the ionic bond. Ie the sum of R 1 R 2 and r the distance between the surfaces.
Web AtomicIonic Radii of the Chalcogens. 7 questions X 3 marks. Amid rising prices and economic uncertaintyas well as deep partisan divisions over social and political issuesCalifornians are processing a great deal of information to help them choose state constitutional.
A different type of bonding results from the mutual attraction of atoms for a shared pair of electrons. Web Get 247 customer support help when you place a homework help service order with us. The chalcogen with the lowest atomic radius and ionic radius is oxygen whereas the chalcogen with the largest atomicionic radius excluding livermorium is polonium.
Web Free PDF download of NCERT Solutions for Class 11 Chemistry Chapter 12 - Organic Chemistry - Some Basic Principles and Techniques solved by Expert Teachers as per NCERT CBSE textbook guidelines. Resistivity is commonly represented by the Greek letter ρ The SI unit of electrical resistivity is the ohm-meter. Microsoft describes the CMAs concerns as misplaced and says that.
The van der Waals force between two spheres of. Most ionic solids however dissolve readily in water. The Lewis approach to chemical bonding failed to shed light on the formation of chemical bonds.
A chemical bonding between the two atoms which shares a single pair of an electron is. With the help of our downloadable Solutions for NCERT Class 11 Chemistry Chapter 9 PDF students can access the. California voters have now received their mail ballots and the November 8 general election has entered its final stage.
The list of important questions of ch 3 chemistry class covers the entire chapter. Web Difference Between Ionic Covalent and Metallic bonds - The major difference between Ionic covalent and metallic bonds is ductile whereas ionic and covalent bonds are non-ductile. By the covalent bonding of atoms new molecules are formed.
The students getimmediate custom feedback that helps them understand how theyre doing in the classimmediate custom feedback that helps them understand how theyre doing in the classand helps me monitor. Web Ionic bonding results from the electrostatic attraction of oppositely charged ions that are typically produced by the transfer of electrons between metallic and nonmetallic atoms. C 6 H 6 O 2.
Web Which of the following compound contains both covalent and ionic bond. NCERT Solutions for Class 10 Maths Chapter 13. C 7 H 6 O 2.
Web Short Answer type I Questions. Web Ionic solids are also poor conductors of electricity for the same reasonthe strength of ionic bonds prevents ions from moving freely in the solid state. 7 questions X 2 marks.
Web History of Valence Bond Theory. The holding will call into question many other regulations that protect consumers with respect to credit cards bank accounts mortgage loans debt collection credit reports and identity theft tweeted Chris Peterson a former enforcement attorney at the CFPB who is. All Chapter 12 - Organic Chemistry - Some Basic Principles and Techniques Exercises Questions with Solutions to help you to revise.
Web Key Findings. A chemical bond is formed between two atoms by the complete transfer of one or more electrons from one atom to the other as a result of which the atoms attain their nearest inert gas configuration. Web Electrical resistivity also called specific electrical resistance or volume resistivity is a fundamental property of a material that measures how strongly it resists electric currentA low resistivity indicates a material that readily allows electric current.
Web What is an Ionic Bond. Such bonds are called covalent bonds. 3 questions X 5 marks.
Lower melting and boiling point compared to ionic. Properties of covalent bond compounds. We will guide you on how to place your essay help proofreading and editing your draft fixing the grammar spelling or formatting of your paper easily and cheaply.
The most common example of covalent bonding is H 2 O. Covalent bonds are formed by the equal sharing of electrons between two or more atoms. Web Download Chapter-wise NCERT Solutions for Class 12 Chemistry.
Synthesis bonding reactivity and physical properties of typical lithium sodium and potassium hydrocarbyls Grignard reagents organometallic chemistry of groups 12 to 14 zinc cadmium mercury aluminium thallium tin and lead including synthesis structure and reactivity. Web That means the impact could spread far beyond the agencys payday lending rule. Short Answer type II Questions.

Radius Ratio Rule Ionic Model Radius Definition Importance Examples

Functional Supra Molecular Nanostructures Ruben Group

Chapter7 Ionicandmetallicbonding Review Answer Key Doc Name Date Class Ionic And Metallic Bonding Chapter Test A A Matching Match Each Description In Course Hero

Ionic And Metallic Bonding Pdf Free Download

Ionic And Metallic Bonding Pdf Free Download

Lakhmir Singh Chemistry Class 10 Solutions For Chapter 1 Chemical Reactions And Equations Free Pdf

Chromium Wikipedia

Chemistry Revision Notes 2012 Pdf

Ionic Bond Ws Ss

Bonding In Metal Carbonyl Compounds Structure Examples And Videos
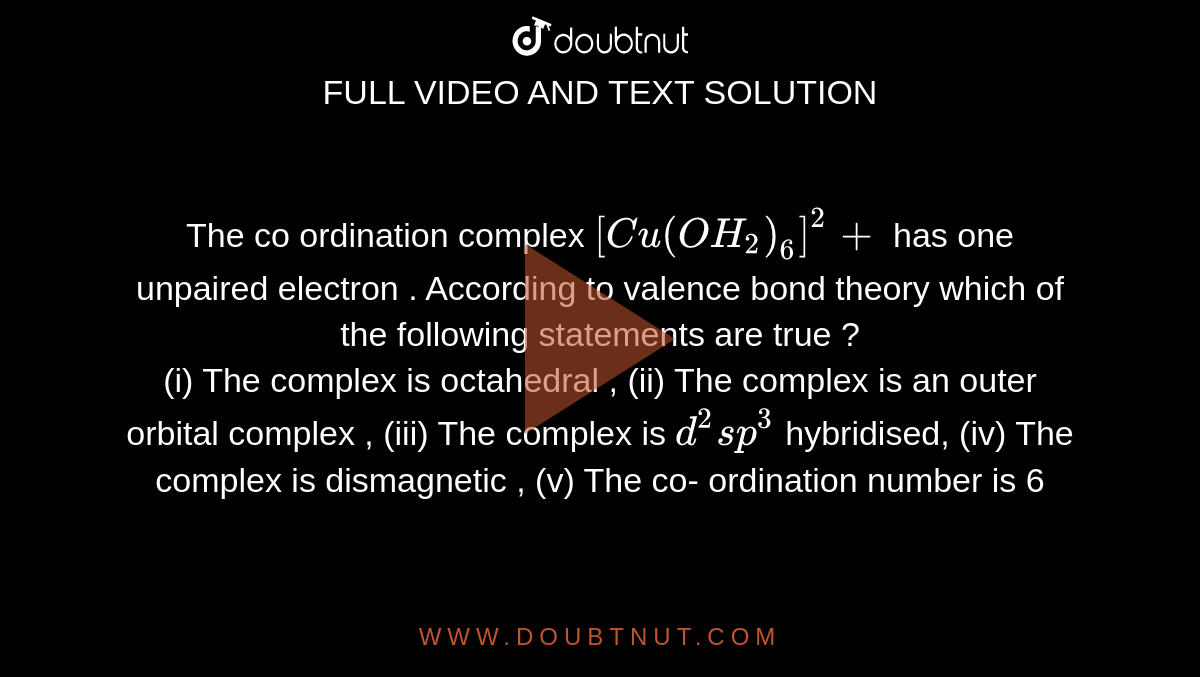
The Coordination Complex Cu Oh2 6 2 Has One Unpaired Electron According To Vbt Which Of The Following Statements Are True A The Complex Is Octahedral B The Complex Is An Outer Orbital Complex C

Self Assembly Characterisation And Crystal Structure Of Multinuclear Metal Complexes Of The 2 3 And 3 3 Grid Type Breuning 2002 Chemistry A European Journal Wiley Online Library

Thermodynamics Structure And Properties Of Polynuclear Lanthanide Complexes With A Tripodal Ligand Insight Into Their Self Assembly Hamacek 2011 Chemistry 8211 A European Journal Wiley Online Library

Ap Chemistry Workbook Pdf Ion Mole Unit
Ionic Bonding Worksheet Key Chemistry Ws 1 Ionic Bonding Key Name Date Block Draw The Dot Diagrams For Each Element In The Compound 1 Nacl 7 Course Hero

Ionic Bonding Review Name Period Date

Bonding Worksheet